- Generality
- Reagent Availability
- Experimental User Friendliness
- Safety and Environmental Impact
-
General Characteristics
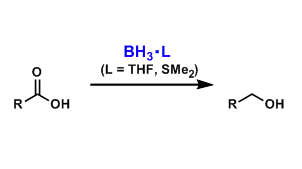
Uncomplexed borane (BH3), which usually exists as diborane (B2H6), is difficult to handle since it is a toxic and pyrophoric gas.
When complexed with THF or dimethylsulfide, borane can exist as monomers and these complexes are sold as solutions that can be dispensed easily. These reagents are used to reduce carboxylic acids to alcohols selectively in the presence of other functional groups such as esters, amides, and nitriles. The reduction of esters, amides, and nitriles becomes possible at higher temperatures.
-
General References
Burg, A. B.; Schlesinger, H. I. J. Am. Chem. Soc. 1937, 59, 780. DOI: 10.1021/ja01284a002
-
Reaction Mechanism
The acyl boronate intermediate acts as an activated ester and undergoes hydride addition twice.

-
Examples
-
Experimental Procedure
-
Experimental Tips
The THF and dimethylsulfide complexes are equally reactive but the latter is less expensive and more stable. The odor of dimethylsulfide, however, is problematic.
-
References
-
Related Books