- Generality
- Reagent Availability
- Experimental User Friendliness
- Safety and Environmental Impact
-
General Characteristics
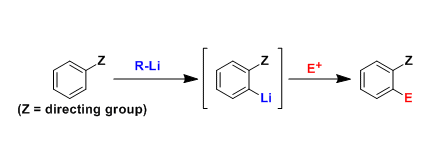
Ortho-selective functionalization of aromatic compounds is possible for the substrates having appropriate directing atoms or groups. Directed ortho metalation (abbreviated as DoM) is one of the most popular ways to synthesize functionalized aromatic compounds.
Although DoM requires a strong base and (often) cryogenic temperature, it is still a valuable reaction in both laboratory and industry because of the highly reliable regioselectivity.
-
General References
Snieckus, V. Chem. Rev. 1990, 90, 879. DOI: 10.1021/cr00104a001
-
Reaction Mechanism
The interaction between the lithium and the electron-rich directing group favors the lithiation at the ortho position.

-
Examples
Lithiation and functionalization of sterically hindered positions is possible. An example is shown below.[1]

Aldehydes react with organolithiums and cannot be used as a directing group. However, in situ protection using a deprotonated chelating diamine allows them to undergo ortho lithiation.[2]

The same concept has been used to achieve consecutive DoMs. Shown below is an application to the synthesis of camptothecin.[3]

-
Experimental Procedure
-
Experimental Tips
-
References
[1] Pansegrau, P. D.; Rieker, W. F.; Meyers, A. I. J. Am. Chem. Soc. 1988, 110, 7178. DOI: 10.1021/ja00229a037 [2] Comins, D. L.; Brown, J. D. J. Org. Chem. 1984, 49, 1078. DOI: 10.1021/jo00180a024 [3] Comins, D. L.; Baevsky, M. F.; Hong, H. J. Am. Chem. Soc. 1992, 114, 10971. DOI: 10.1021/ja00053a049
-
Related Books